Antidepressant Efficacy and Tolerability of Ketamine and Esketamine a Critical Review
Introduction
Ketamine has been studied and used for psychiatric purposes for over twenty years (i). Its rapid and robust antidepressant event has been reproduced across numerous studies past significantly decreasing the severity of depression, achieving substantial rates of response and remission even for patients that were not-responsive to previous treatments (ane–v).
Over the years, interest in ketamine, its racemic chemical compound and enantiomers [i.eastward., S-ketamine (esketamine) (6) and R-ketamine (arketamine) (7)], has increased in the literature since 2016. This led to FDA approval of intranasal (IN) esketamine for treating depression in 2019 (viii, nine).The pharmacokinetic features of ketamine permit for its administration past numerous routes including intravenous (IV) (1–5), subcutaneous (SC) (10), intranasal (IN) (8, 9), oral (xi), sublingual (12), and intramuscular (IM) (xiii). That said, the optimal route of administration has all the same to be defined.
Although the efficacy of ketamine for treatment resistant depression (TRD) has been demonstrated using unlike routes of assistants, nearly studies have adopted the IV, and more recently IN road. The classical 4 protocol infuses a dose of 0.v mg/kg of racemic ketamine over 40 min (one). This protocol requires the use of an infusion pump every bit well every bit skilled nursing staff and extended medical supervision, resulting in relatively elevated costs beyond both infrastructure and human resources. In comparison, IN esketamine does not require an infusion pump and requires less resources, but has an estimated cost of between U$5,664 and 8,142 for the first month of handling (14).
The SC route has been proposed to exist a more than convenient, cheaper and less complex route of assistants and has been suggested to exist equally effective as and perhaps safer than the Iv administration (6). This road has also been used to treat other conditions since 1975 (15).
Although in that location is still controversy regarding an exact dose-response human relationship, both dosage and route of administration directly influence the efficacy and tolerability of ketamine and its enantiomers. SC is an easier and more convenient route, not requiring equipment and skilled staff. This is especially relevant in developing countries that struggle to optimize scarce resources. The chief aim of this systematic review is to assess the efficacy, tolerability and feasibility of SC ketamine and its enantiomers for the handling of low.
Methods
The written report protocol was registered on the International Prospective Register of Systematic Reviews database (PROSPERO; registration number: CRD42019137434) and adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16).
Search Strategy and Information Sources
A systematic literature search was carried out using PubMed/MEDLINE, EMBASE and Web of Science, from inception upwards until March 12, 2021. The search terms employed were ketamine AND subcutaneous AND depress*.
Studies Choice and Data Extraction
Later on excluding duplicates, two authors (VC and LC) independently reviewed the abstracts to check eligibility. Initial selected manufactures were retrieved in full text to apply inclusion/ exclusion criteria and confirm eligibility. Disagreements were discussed with a third writer (RF) and resolved past consensus.
Inclusion/Exclusion Criteria
To be considered eligible, each accepted study must had included patients with a diagnosis of Major Depressive Disorder or Major Depressive Episode (unipolar or bipolar). SC racemic ketamine, esketamine or arketamine must take been used in at least one session. Studies using ketamine associated with other interventions such as ECT were considered ineligible. We accepted clinical trials, retrospective studies, and case reports. We accepted all languages and there were no limits regarding age of participants.
Data Analysis and Summary Measures
The above criteria identified 12 acceptable studies. Given that we establish so few studies with heterogeneous methods, a meta-analysis was not considered appropriate and a narrative review was performed.
Thus, here we provide a synthesis of these data including characteristics of participants, dosing, study blueprint, and findings. Table 1 (Take a chance of bias) was fabricated employing Cochrane take a chance of bias tool (19), Table 2 (Mood outcome) included a summary of the post-obit characteristics: methods, participants, number of subjects, dose, number of sessions, remission and response, while Tabular array 3 (Safety and Tolerability cess) the tools employed by each study to assess Safety and Tolerability were spared in the post-obit categories: psychiatric or psychotomimetic, neurological or cognitive, cardiovascular, other.

Tabular array i. Run a risk of bias.

Table 2. Mood effect.

Tabular array 3. Safety and tolerability assessment.
Take chances of Bias in Individual Studies
The take chances of bias was accessed by 2 authors using a take chances of bias cess based on a modified Cochrane risk of bias tool (19). Disagreements were discussed with a third author and resolved by consensus. Each study received a score of low, high or unclear risk of bias in each category.
Results
Studies Selection
1 hundred fifty-nine studies were found via our electronic database search: Pubmed/MEDLINE (northward = 59), EMBASE (north = 53) and Web Of Science (n = 47). Lxxx articles were selected after removing duplicates and abstracts were accessed. Xi articles were selected after removing studies that did not run across inclusion/ exclusion criteria and were retrieved in full text. One article was retrieved from the references section of one identified study. Thus, 12 articles are included in this review (Effigy 1).
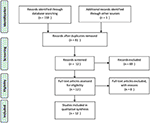
Effigy i. PRISMA flow diagram of search strategy.
Risk of Bias Inside Studies
Details of the take chances of bias assessment are provided in Table ane, information technology was excluded from this assessment example reports and chart review. The study conducted by Loo et al. (18) was classified as low adventure of bias for allotment concerning agile placebo vs. ketamine. However, no mention was made regarding strategies for blinding the road of administration, IV, IM or SC, which increases the take a chance of bias.
Synthesized Findings
Of the selected articles, 2 were randomized clinical trials (17, eighteen) v were case-reports (21, 22) and 5 were retrospective studies (20, 26–29) (see Table 2). From the retrospective studies, four of them (26–29) were from the intervention in the same group of patients and, thus, volition be presented together in Tables ii, 3.
Mood Issue
In a randomized double-bullheaded multiple-crossover placebo-controlled trial followed by an open label phase, George et al. (17) enrolled 16 unipolar or bipolar depressed patients aged > threescore years. Patients showed an insufficient response to at least ane treatment during the current mood episode and had MADRS ≥ 20. All patients remained on a stable dose of psychotropic medications during the written report. In the randomized clinical trial (RCT) phase, patients received weekly SC racemic ketamine at a progressive dose, starting at 0.1 mg/kg and increasing 0.1 mg/kg, each session, up to 0.5 mg/kg. The protocol was stopped when the patient reached remission (MADRS <ten) at 24-hour interval 7 afterward last administration or at the fifth session, with 0.5 mg/kg. For not-remitters, the dose was increased up to 0.5 mg/kg. 7 participants (43,75%) met the benchmark for remission, 1 week later on terminal administration (ketamine doses 0.1 mg/kg: Northward = 1; 0.iii mg/kg: N = 1; 0.4 mg/kg: N = three; and 0.5 mg/kg: N = 2) and were followed until relapse. Seven participants (43.75%) received up to 0.5 mg/kg and did non remit. Two patients dropped out due to unrelated illness. MADRS scores significantly decreased for 0.2 mg/kg (p < 0.01), 0.iii mg/kg (p < 0.001), and 0.four mg/kg (p < 0.001) doses, but not for the 0.ane mg/kg (p = 0.06) dose, as compared with placebo (midazolam). In the open label phase, patients received 12 administrations of the dose of remission or 0.5 mg/kg if remission was not attained with lower doses. In this stage, 12 patients, 5 RCT remitters who relapsed and 7 RCT non-remitters, received SC racemic ketamine twice weekly for iv weeks and and then weekly for 4 weeks. For the seven RCT non-remitters, two attained remission with repeated treatments (0.5 mg/kg).
Loo et al. (18) conducted a double-blind, placebo-controlled trial comparison routes of administration of racemic ketamine: IM (N = five), IV (N = 4) and SC (North =6). They included 15 patients aged > sixty years with a diagnosis of major depression disorder (MDD), a MADRS ≥ 20 and an bereft therapeutic response to at to the lowest degree i antidepressant trial. The patients were allowed to maintain psychotropic medications in stable doses during the protocol. The protocol used a progressive ketamine dose ranging from 0.ane to 0.5 mg/kg, with intervals of at least 1 week and increments of 0.1 mg/kg a calendar week, if the patient did not reach remission past the 7th solar day (MADRS <10). The employ of Four ketamine was double-blinded with administration of active placebo (midazolam) at 0.01 mg/kg. Twelve patients reached remission [75% (Iv), 60% (IM) and 100% (SC)] and all three routes of administration resulted in comparable antidepressant furnishings.
We identified two case reports examining response to SC racemic ketamine (21, 22). In one case (22), a single dose of 0.5 mg/kg significantly reduced symptoms of depression and feet. In the other case (21), the patient showed a remission for 5 months after 2 doses (0.i and 0.ii mg/kg), dosed at to the lowest degree a week apart. She and so received 12 more doses (offset viii, dosed twice-weekly, adjacent four, weekly) of 0.2 mg/kg and remained remitted for x weeks after the last injection.
Nosotros identified three case reports using SC esketamine. In the first report of SC esketamine, Costa et al. (23) reported remission of symptoms in a 75 year-old patient with bipolar depression [who was resistant to electroconvulsive therapy (ECT)] after a unmarried session of SC esketamine at 0.five mg/kg. Barbosa et al. (24), in a setting of palliative intendance, reported a case of remission afterward 3 sessions (of a total of iv, dosed twice-weekly) with esketamine from 0.5 to 0.75 mg/kg. The tertiary case report administered SC esketamine in a 76 year-old patient with depression and Alzheimer Disease. The patient showed improvement in depressive symptoms - dose ranged from 0.5 to 0.75 mg/kg in three sessions (viii in total, dosed twice-weekly) (25).
The identified nautical chart review (twenty) reported two patients (among a grouping of 31 patients) that received a single dose of SC racemic ketamine at 0.five mg/kg. Unfortunately, the results were not presented separately from other patients (n = 29) who had received oral ketamine. The entire group (n = 31) showed meaning global improvement as measured using the Clinical Global Impression (CGI).
In a retrospective existent world study, Lucchese et al. (26) described 70 patients aged > fifteen years with a diagnosis of MDD (39 patients) or BD (31 patients), who showed no response to ≥ 2 treatment in a electric current episode and a MADRS ≥ 25. Patients received half-dozen sessions of SC esketamine, dosed weekly, starting with dosage of 0.v mg/kg. The dosage was increased by 0.75 or 1 mg/kg if the patient did not reply to the previous dosage (decrease in MADRS by ≥ 50%) one week later. 80 per centum of patients had non responded to five previous antidepressant treatments and the current episode was longer than 2 years for 70%. Fifty one (72.9%) patients had dosage titrated to one mg/kg of esketamine. 30-five (50%) patients responded. Anhedonia was evaluated separately by detail eight of the MADRS (inability to feel), and comeback was observed 24 h afterward the first session (t = 4.007; p < 0.001), with a further reduction in anhedonia scores following repeated infusions. No significant differences were observed betwixt MDD and BD (28).
In this same group of lxx patients, the probability of response after each of the first four sessions of esketamine was estimated by subconscious Markov modeling. The probability of a non-responder to become a responder afterwards an injection was 17.30%, while the probability for a "responder" to remain as a "responder" was 95% (27).
Rubber and Tolerability of SC Road
Loo et al. (18) and George et al. (17) assessed tolerability with positive symptom items from the Brief Psychiatric Rating Calibration (BPRS) (30), item 1 (Elevated mood) of the Immature Mania Rating Scale (YMRS) (31) and the Clinician Administered Dissociative Symptoms Scale (CADSS) (32). They took these measurements at baseline, 40 min later injection and later 4 h. Other side-effects were accessed with a modified version of the SAFTEE scale (33). Orientation and unproblematic and complex reaction times were measured at baseline and 4 h after injection. Hemodynamic furnishings were evaluated with measurement of heart rate and blood pressure at v, 10, 30, threescore, and 240 min after administration. Liver part was as well measured by George et al. (17) before and later on RCT.
Loo et al. (18) found dissociative psychotomimetic effects direct related to dose. The IV group had higher superlative scores when evaluated forty min afterwards dosage. Symptoms included mild depersonalization, derealization, contradistinct body perception and contradistinct time perception. Items from the BPRS and Item 1 of YMRS did not show mania symptoms at observed endpoints. Other reported side-effects were fatigue, lightheadedness, dizziness, blurred vision and emotional lability. Increases in heart rate and blood force per unit area did not exceed 120%. The peak of reported effects were between 10 and 15 min, resolving spontaneously between 30 and 60 min.
George et al. (17) besides observed a dose–response relationship for dissociative psychotomimetic furnishings. Symptoms reported were: mild perceptual disturbance (colors or sounds seemed different), derealization, altered body perception, and contradistinct time perception. Peak furnishings occurred 10–fifteen min after injection. BRPS and YMRS showed no emergent psychiatric symptoms at whatsoever time indicate and there were no clinically meaning changes compared to the midazolam status. Transient increases in systolic and diastolic claret force per unit area were occasionally observed, with height incidence 4 h after administration and increases in heart charge per unit only exceeded 120% four times, but did non exceed 131.five%.
Other side-furnishings reported were: Palpitations, Flushing, Lightheadedness/ Dizziness, Fatigue/ Sleepiness/ Poor Concentration/ Feeling Vague (Spaced Out), Paresthesia, Nausea, Dry Oral fissure, Blurred Vision/Diplopia, restlessness, and headache. All reported side-effects resolved within thirty–60 min without the need for medical intervention. All participants were oriented at 4 h post-treatment. Liver part from fourteen patients was inside normal limits except for discrete elevations of transaminases in ii patients after RCT.
In the open label phase minimal increases in center rate, blood force per unit area, and CADSS scores were observed with no evidence of cumulative increases. Dizziness (north = 2), numbness (Northward = 2), headache (north = one), and urge to urinate slightly more ofttimes (northward = 1) were reported and resolved spontaneously. One patient (of eight) showed a slight increment in transaminases later on the course.
Iglewicz et al. (20) assessed side effects with ratings on the Clinical Global Impression (CGI) scale based on a palliative care team charting at baseline and post-ketamine dosing. The results from SC ketamine were presented together with an oral dosage and showed that 3 (13.6%) patients had only 1 side issue, half dozen (27.3%) had up to iii psychiatric side effects and 13 (59.ane%) had no side effects. The side furnishings reported were: disorientation [Due north = 7 (45.5%)], hallucination [due north = iv (18.2%)], sedation [North = 4 (18.2%)], insomnia [N = i (four.5%)], delusions [N = ane (iv.v%)], and anxiety [N = 1 (four.five%)].
Del Sant et al. (29) described in a sample of 70 patients [same from Lucchese et al. (26), Fava et al. (27) and Delfino et al. (28)] that SC esketamine was well-tolerated for doses of 0.five, 0.75, and 1 mg/kg. Systolic Blood Pressure (SBP) increased well-nigh iv.87 while Diastolic Blood Pressure (DBP) increased five.54 mmHg within thirty–45 min, returning to baseline within 120 min. No meaning heart charge per unit changes were observed. xiv/70 patients had SBP > 180 mmHg and/or a DBP >110 mmHg. Other assessments of tolerability in this sample were fabricated but the manuscript is still in preparation past authors.
In the case reports ketamine and esketamine were by and large well-tolerated, with reports of mild lightheadedness and blurred vision (21), transient summit in blood pressure and eye rate (23, 24) and abdominal pain (24).
The tolerability assessment is presented in Table 3. The measurements that were near often employed were the BPRS, YMRS, CADSS, SAFTEE, middle charge per unit and blood pressure.
Other Findings
The ketamine blood concentration was assessed by Loo et al. (18). Blood samples were obtained at baseline, and and so 5, fifteen, 30, 120, and 240 min later on IV dosing and fifteen, 30, 120, and 240 min after IM/SC injection. Plasma concentrations recorded afterwards IV showed a acme between 350 and 400 ng/ml (dose of 0.5 mg/Kg), with a peak below 200 ng/ml in SC route. Plasma concentrations were linearly correlated with the ketamine dosage (IV, r = 0.88, P < 0.001; IM, r = 0.92, P < 0.001; SC, r = 0.86, P < 0.001) as well as CADSS scores at twoscore min (r = 0.44, P = 0.001).
No data were presented regarding costs or cost-effectiveness of the SC road in low. The only estimated price was presented by Lucchese et al. (26) of one esketamine ampoule (50 mg/mL, 2 mL) at BRL R$15.00 (~US$2.70) for approximately two dosages.
Discussion
In this review we found twelve manufactures examining SC racemic ketamine and esketamine in low. The results upward to now are promising, with efficacy comparable to Iv ketamine and only transitory side effects. However, many limitations, such as a relatively small number of patients and patients from the same sample, limit the findings.
The utilize of SC ketamine has been described in humans since 1975 (15) and has been explored mainly for pain (fifteen, 34–36) perioperative analgesia (37–39) and anesthesia (10). Javid et al. (10) found that the SC and 4 routes similarly produced a dissociative consciousness in a laparoscopic procedure. They proposed that the SC route is safer, since some patients in the IV group lost their power to cooperate and experienced mild hallucinations. The SC dose used, however, was 0.6 mg/kg while the 4 protocol appeared to exist in bolus. We annotation that these dosages are not common in most protocols used for low. In another study (38) comparing IV to SC routes for pain relief after tonsillectomy, at a SC dosage of 0.5 mg/kg for both routes and the IV administration performed in bolus, the results were similar for both routes. For depression, the get-go trial conducted by Berman et al. (1) used an IV protocol that has been widely replicated in studies addressing the antidepressant effect of ketamine. Their protocol consisted of 0.five mg/kg of ketamine administered intravenously with an infusion pump over xl min.
As discussed earlier, the use of an SC route is mainly motivated by ease of use and possible reduction of costs in both equipment and human resources. The cost-effectiveness of ketamine was evaluated in previous studies for traumatic injuries (36) and Chiari disease (39). Despite potentially important cost-do good advantages associated with the use of an SC route for treating depression, the present review showed that, up to now, only the costs of an esketamine ampoule (50 mg/mL, 2 mL) has been presented in the literature, costing BRL R$15.00 (~US$two.seventy) for approximately two dosages (26). For comparison, the costs estimated for IN esketamine range from U$5,664 to eight,142 for the showtime calendar month of handling, while Four racemic ketamine costs in the U.s.a. range from U$500 to i,000 per session (14). This toll would be prohibitive in developing countries.
In this review, we found encouraging results for the SC use of ketamine in the treatment of depression. Favorable results regarding both efficacy and prophylactic were reported in case reports (21–25). Data from retrospective studies (20, 26–29) and 2 clinical trials (17, 18) also confirmed efficacy and a solid tolerability contour associated with SC administration of ketamine for depression. Considering the paucity of data, we will discuss efficacy and tolerability of these results qualitatively, keeping in heed the limited nature of the data at hand.
Efficacy
To date, the available data support the efficacy of the SC road. Loo et al. (18) demonstrated that all patients who received SC ketamine showed remission or response (100%) at to the lowest degree at one endpoint with a dosage below 0.v mg/kg. Despite the small number of patients, the results were impressive. In the dose titration report conducted by George et al. (17) involving elderly patients with MDD and BD, eleven (68.eight%) of 16 patients responded and nine (56.25%) remitted, some with a dosage even lower than 0.five mg/kg. For comparison, we annotation that two previous studies using the 4 route reported remission rates of 23% (v) and 29% (2). These data are comparable with results of other studies using the IV route: fifty% of response (ane), 71% of response and 29% of remission (2), 64% of response (3), 59% of response and 23% of remission (five). One study using IN esketamine documented a 69.3% rate of response (9) Thus, despite the paucity of available data, results found with SC ketamine and esketamine are promising.
Our ability to evaluate the dose-response effect of the SC road measured in the two trials with ketamine, as well as the four retrospective studies (26–29) and 2 example-reports (24, 25) with esketamine in comparison to IV studies is limited since the latter studies used stock-still doses. Nonetheless, findings from dose-titration studies appear promising with patients remitting following low doses. This volition be important for patients that are vulnerable to side effects, including the elderly and those with medical comorbidities. Dose titration was also used in an IV racemic ketamine protocol (iv), with doses of 0.1, 0.2, 0.5, and 1 mg/kg. Results indicated no clear or consequent efficacy for the dosages of 0.1 and 0.2 mg/kg.
Condom and Tolerability
Loo et al. (eighteen) and George et al. (17) used similar parameters to assess tolerability [except for the liver part assessed in the latter (14)], and were in agreement with the literature (40). Loo et al. (18) establish better tolerability in the SC group, which had lower scores in dissociative psychotomimetic symptoms (CADSS) as compared to the Four group. Notwithstanding, no significant master effect for whatever route was found. The Iv administration was performed in ii–5 min, which may increment psychotomimetic symptoms. We note that blood concentration was nearly double for Four road compared to both SC and IM routes. Blood concentrations using a 0.v mg/kg Iv protocol in 40 min have been reported to be around 200 ng/dL (41) similar to that found for the SC route in the study of Loo et al. (eighteen). Too, the instruments used to assess tolerability were in agreement with the literature (40). Thus, to date, SC ketamine and esketamine demonstrate a solid tolerability profile, with few and transient side effects, similar to the IV (40) and IN routes (8, nine). In addition, considering that 2 example reports (xvi, 24) and a retrospective study (twenty) were performed in the context of palliative intendance, and some other trial involved elderly patients (17), most of the data considered hither were from patients with medical comorbidities.
Future Perspectives
Since the piece of work of Berman et al. (1), many studies accept aimed to appraise the outcome of ketamine in MDD and BD. Since 2016, esketamine has been investigated since it is a more potent adversary of the NMDA receptor. A contempo systematic review showed that racemic ketamine, compared to esketamine, demonstrated greater overall response rates (RR = iii.01 vs. RR = i.38), remission rates (RR = iii.70 vs. RR = 1.47), besides as lower dropouts (RR = 0.76 vs. RR = one.37) (42). Knowing the heterogeneity of studies, including that all esketamine studies were IN and ketamine studies were Four, should nosotros render to racemic ketamine or explore esketamine more? Recently, a study using arketamine showed promising effects without dissociative effects (7). The question of whether racemic ketamine or its enantiomers are amend for each case remains an open question.
Furthermore, the all-time route of administration is still an open question more than 20 years later. IV ketamine was the start protocol studied, with all its advantages and disadvantages. IN esketamine appeared to solve some of these problems with practical dispositive and feasibility, leading to its approval by several regulatory agencies throughout the world. However, there is a possible bear upon on efficacy when using IN esketamine (42). The IM and SC routes appear to be somewhat practical, but as we establish in this review regarding SC, the studies in the literature are still few. This review has shown that at that place remains a lack of a robust trials comparing these routes, or even comparison racemic ketamine to its enantiomers.
Limitations
Five of the twelve studies we reviewed were case reports, and some other five were retrospective studies. In the written report conducted by Iglewicz et al. (20), the results for SC ketamine were not described separately and the depression criteria was non clear. Likewise, in the McNulty and Hahn (22) report, the depression diagnosis criteria was besides non clear.
The report by Galvez et al. (21) is a case report of a patient that took part in the study published past George et al. (17). The trials conducted by Loo et al. (18) and George et al. (17) enrolled a minor number of patients (15 and 16 patients, respectively). In addition, George et al. (17) studied subjects that were ≥threescore years of age, which is not representative of the general population, and patients with BP beyond MDD. In addition, iii (17, 18, 21) of five studies were performed on the same group of individuals.
The retrospective studies by Fava et al. (27), Del Sant et al. (29), Delfino et al. (28), and Lucchese et al. (26) were from the same sample and from the same site, which also limits the findings.
Conclusion
There are deficient information on SC racemic ketamine and esketamine for depression, and there is no study comparing this road with the most usually used 4 or IN protocols. As well, there are no data addressing the price or cost-effectiveness of this route. The SC road may be particularly appealing to developing countries, where resources scarcity is often a major limiting factor. Available data suggest that SC ketamine and esketamine is a promising alternative for TRD, showing solid efficacy and tolerability. Future randomized clinical trials comparing routes of administration focusing on efficacy, tolerability, pharmacokinetics, cost-effectiveness and long term follow upwards assessments are needed.
Data Availability Statement
All datasets generated for this report are included in the article/supplementary material.
Writer Contributions
VC, LC, and RF designed the study. VC and LC conducted literature search and data extraction. Disagreements were discussed with RF and resolved by consensus. VC and LC wrote the beginning typhoon. AL, EH, EM, and RF contributed to data interpretation and revised the manuscript critically, contributing to many aspects of the discussion. All authors contributed to and approved the final version of the manuscript and agreed to exist accountable for all aspects of the work in ensuring that questions related to the accurateness or integrity of any office of the work are appropriately investigated and resolved.
Conflict of Interest
AL has received consulting fees from Hoffmann–La Roche, Genentech, Janssen Pharmaceutical, Daiichi Sankyo, Cristalia Produtos Químicos eastward Farmacêuticos, Pfizer, Mantecorp Indústria Química e Farmacêutica, Libbs Farmacêutica, FQM Farma, and Sanofi-Aventis over the last 24 months and has received research fees from Janssen Pharmaceutical, Eli Lilly, Novartis, Biophytis, Celltrion, Azidus, H. Lundbeck A/South, Servier Laboratories, Hoffman-La Roche, FQM Farma, and Forum Pharmaceuticals.
The remaining authors declare that the enquiry was conducted in the absence of whatsoever commercial or financial relationships that could exist construed equally a potential disharmonize of involvement.
References
1. Berman R, Cappiello A, Anand A, Oren D, Heninger 1000, Charney D, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. (2000) 47:351–4. doi: x.1016/S0006-3223(99)00230-9
PubMed Abstract | CrossRef Total Text | Google Scholar
2. Zarate C, Singh J, Carlson P, Brutsche N, Ameli R, Luckenbaugh D, et al. A randomized trial of an n-methyl-d-aspartate adversary in treatment-resistant major depression. Arch Gen Psychiatry. (2006) 63:856. doi: 10.1001/archpsyc.63.8.856
PubMed Abstract | CrossRef Total Text | Google Scholar
3. Murrough J, Iosifescu D, Chang L, Al Jurdi R, Green C, Perez A, et al. Antidepressant efficacy of ketamine in handling-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. (2013) 170:1134–42. doi: 10.1176/appi.ajp.2013.13030392
PubMed Abstract | CrossRef Total Text | Google Scholar
iv. Fava M, Freeman Thou, Flynn M, Judge H, Hoeppner B, Cusin C, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. (2018) 25:1592–603. doi: 10.1038/s41380-018-0256-5
PubMed Abstruse | CrossRef Full Text | Google Scholar
v. Phillips J, Norris Southward, Talbot J, Birmingham M, Hatchard T, Ortiz A, et al. Unmarried, repeated, and maintenance ketamine infusions for handling-resistant low: a randomized controlled trial. Am J Psychiatry. (2019) 176:401–9. doi: x.1176/appi.ajp.2018.18070834
PubMed Abstract | CrossRef Total Text | Google Scholar
6. Singh JB, Fedgchin M, Daly East, Xi L, Melman C, De Bruecker G, et al. Intravenous esketamine in adult treatment-resistant low: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. (2016) eighty:424–31. doi: 10.1016/j.biopsych.2015.ten.018
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Leal GC, Bandeira ID, Correia-Melo FS, Telles M, Mello RP, Vieira F, et al. Intravenous arketamine for treatment-resistant low: open-label pilot study. Eur Arch Psychiatry Clin Neurosci. (2021) 271:577–82. doi: 10.1007/s00406-020-01110-five
PubMed Abstruse | CrossRef Full Text | Google Scholar
8. Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with handling-resistant depression: a randomized clinical trial. JAMA Psychiatry. (2019) 08560:one. doi: ten.1001/jamapsychiatry.2019.1189
PubMed Abstract | CrossRef Full Text | Google Scholar
ix. Popova V, Daly E, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant low: a randomized double-blind active-controlled study. Am J Psychiatry. (2019) 176:428–38. doi: 10.1176/appi.ajp.2019.19020172
PubMed Abstract | CrossRef Full Text | Google Scholar
ten. Javid Yard, Rahimi Chiliad, Keshvari A. Dissociative conscious sedation, an culling to general anesthesia for laparoscopic peritoneal dialysis catheter implantation: a randomized trial comparing intravenous and subcutaneous ketamine. Periton Dial Int. (2010) 31:308–xiv. doi: 10.3747/pdi.2010.00110
PubMed Abstract | CrossRef Total Text | Google Scholar
11. Rosenblat J, Carvalho A, Li Grand, Lee Y, Subramanieapillai M, McIntyre R. Oral ketamine for depression. J Clin Psychiatry. (2019) 80:18r12475. doi: 10.4088/JCP.18r12475
CrossRef Full Text | Google Scholar
12. Lara D, Bisol L, Munari L. Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol. (2013) sixteen:2111–7. doi: 10.1017/S1461145713000485
PubMed Abstract | CrossRef Total Text | Google Scholar
13. Cusin C, Hilton 1000, Nierenberg A, Fava Thou. Long-term maintenance with intramuscular ketamine for handling-resistant bipolar II depression. Am J Psychiatry. (2012) 169:868–9. doi: 10.1176/appi.ajp.2012.12020219
PubMed Abstract | CrossRef Total Text | Google Scholar
14. Bahr R, Lopez A, Rey JA. Intranasal esketamine (SpravatoTM) for use in handling-resistant depression in conjunction with an oral antidepressant. P T. (2019) 44:340–75.
PubMed Abstract | Google Scholar
xv. Weber W, Jawalekar K, Jawalekar S. The event of ketamine on nerve conduction in isolated sciatic nerves of the toad. Neurosci Lett. (1975) 1:115–20. doi: 10.1016/0304-3940(75)90055-5
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) half dozen:e1000100. doi: 10.1371/periodical.pmed.1000100
PubMed Abstruse | CrossRef Full Text
17. George D, Gálvez Five, Martin D, Kumar D, Leyden J, Hadzi-Pavlovic D, et al. Pilot randomized controlled trial of titrated subcutaneous ketamine in older patients with treatment-resistant depression. Am J Geriatr Psychiatry. (2017) 25:1199–209. doi: 10.1016/j.jagp.2017.06.007
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Loo C, Gálvez V, O'Keefe East, Mitchell P, Hadzi-Pavlovic D, Leyden J, et al. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand. (2016) 134:48–56. doi: 10.1111/acps.12572
PubMed Abstract | CrossRef Total Text | Google Scholar
19. Higgins J, Altman D, Gotzsche P, Juni P, Moher D, Oxman A, et al. The Cochrane Collaboration'south tool for assessing gamble of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Iglewicz A, Morrison K, Nelesen R, Zhan T, Iglewicz B, Fairman N, et al. Ketamine for the treatment of depression in patients receiving hospice care: a retrospective medical record review of thirty-ane cases. Psychosomatics. (2015) 56:329–37. doi: 10.1016/j.psym.2014.05.005
PubMed Abstruse | CrossRef Full Text | Google Scholar
21. Gálvez V, O'Keefe E, Cotiga L, Leyden J, Harper S, Glue P, et al. Long-lasting effects of a unmarried subcutaneous dose of ketamine for treating melancholic depression: a case study. Biol Psychiatry. (2014) 76:e1–two. doi: 10.1016/j.biopsych.2013.12.010
PubMed Abstruse | CrossRef Full Text | Google Scholar
22. McNulty JP, Hahn 1000. Compounded oral ketamine. Int J Pharm Compd. (2012) sixteen:364–8.
Google Scholar
23. Costa L, Cavenaghi V, Bassit D, Folquitto J, Hirata E, Fraguas R. Efficacy, condom, and tolerability of a single subcutaneous dose of ketamine to treatment-resistant depression in the elderly: a case report. Int Psychogeriatr. (2019) 31(Suppl. one):94−5. doi: 10.1017/S1041610219001339
CrossRef Full Text | Google Scholar
24. Barbosa MG, Delfino RS, Sarin LM, Jackowski AP. Repeated subcutaneous esketamine administration for depressive symptoms and pain relief in a terminally ill cancer patient: a case study. Palliat Med. (2020) 34:822–v. doi: ten.1177/0269216320910351
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Rocha FL, Cunha UGV, Paschoalin RC, Hara C, Thomaz DP. Utilise of subcutaneous ketamine to rapidly improve severe treatment-resistant low in a patient with Alzheimer'due south illness. Int Clin Psychopharmacol. (2021) 36:104–5. doi: 10.1097/YIC.0000000000000334
PubMed Abstruse | CrossRef Full Text | Google Scholar
26. Lucchese Air-conditioning, Sarin LM, Magalhães EJM, Del Sant LC, Puertas CB, Tuena M, et al. Repeated subcutaneous esketamine for treatment-resistant low: impact of the degree of treatment resistance and anxiety comorbidity. J Psychopharmacol. (2021) 35:142–nine. doi: 10.1177/0269881120978398
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Fava VAR, Sarin LM, Lucchese Air-conditioning, Del Sant L, Magalhães E, Delfino RS, et al. The probability of response after each subcutaneous injection of esketamine in treatment-resistant depression. Rev Psiquiatr Salud Ment. (2020). doi: ten.1016/j.rpsm.2020.10.003
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Delfino RS, Del-Porto JA, Surjan J, Magalhães E, Del Sant LC, Lucchese AC, et al. Comparative effectiveness of esketamine in the handling of anhedonia in bipolar and unipolar depression. J Affect Disord. (2021) 278:515–8. doi: x.1016/j.jad.2020.09.056
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Del Sant LC, Sarin LM, Magalhães EJM, Lucchese AC, Tuena MA, Nakahira C, et al. Furnishings of subcutaneous esketamine on blood pressure and heart rate in treatment-resistant depression. J Psychopharmacol. (2020) 34:1155–62. doi: ten.1177/0269881120922955
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Bremner J, Krystal J, Putnam F, Southwick South, Marmar C, Charney D, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Traumatic Stress. (1998) 11:125–36. doi: 10.1023/A:1024465317902
PubMed Abstract | CrossRef Total Text | Google Scholar
34. Mercadante S, Lodi F, Sapio M, Calligara Grand, Serretta R. Long-term ketamine subcutaneous continuous infusion in neuropathic cancer pain. J Hurting Sympt Manag. (1995) ten:564–eight. doi: 10.1016/0885-3924(95)00102-5
PubMed Abstract | CrossRef Total Text | Google Scholar
35. Backonja 1000, Arndt Thousand, Gombar 1000, Check B, Zimmermann M. Response of chronic neuropathic pain syndromes to ketamine: a preliminary study. Hurting. (1994) 56:51–7. doi: 10.1016/0304-3959(94)90149-X
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Ketamine for adult patients who accept suffered painful and traumatic injuries: a review of clinical effectiveness cost-effectiveness safety and guidelines. Can Agency Drugs Technol Wellness. (2014) 23:456–9.
37. Gurnani A, Sharma PK, Sethi AK. Subcutaneous infusion of ketamine and morphine for relief of postoperative pain: a double-blind comparative study. Ann Acad Med. (1994) 23:456–nine.
PubMed Abstruse | Google Scholar
38. Javid K, Hajijafari M, Hajipour A, Makarem J, Khazaeipour Z. Evaluation of a low dose ketamine in post tonsillectomy pain relief: a randomized trial comparing intravenous and subcutaneous ketamine in pediatrics. Anesthesiol Pain Med. (2012) 2:85–nine. doi: ten.5812/aapm.4399
PubMed Abstract | CrossRef Full Text | Google Scholar
39. McDowell M, Alhourani A, Pearce-Smith B, Mazurkiewicz A, Friedlander R. Cost-effectiveness of postoperative ketamine in chiari decompression. Earth Neurosurg. (2018) 110:e599–604. doi: 10.1016/j.wneu.2017.11.061
PubMed Abstract | CrossRef Full Text | Google Scholar
40. Short B, Fong J, Galvez V, Shelker W, Loo C. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. (2018) 5:65–78. doi: 10.1016/S2215-0366(17)30272-9
PubMed Abstract | CrossRef Total Text | Google Scholar
41. Zarate C, Brutsche N, Laje Chiliad, Luckenbaugh D, Venkata Southward, Ramamoorthy A, et al. Relationship of ketamine's plasma metabolites with response, diagnosis, and side effects in major low. Biol Psychiatry. (2012) 72:331–viii. doi: x.1016/j.biopsych.2012.03.004
PubMed Abstract | CrossRef Total Text | Google Scholar
42. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. (2021) 278:542–55. doi: ten.1016/j.jad.2020.09.071
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.513068/full
0 Response to "Antidepressant Efficacy and Tolerability of Ketamine and Esketamine a Critical Review"
Post a Comment